Blockchain‑enabled remote patient monitoring platform advances secure, real‑time data utilization and AI‑assisted clinical insights for next‑generation care and research
AKT Health Co., Ltd., a leading data-driven life science consulting and health-tech company, today announced that its Software as a Medical Device (SaMD) platform “Impakt Health” has received Class II medical device certification from Japan’s Pharmaceuticals and Medical Devices Agency (PMDA). This approval establishes Impakt Health as a blockchain-integrated SaMD platform certified for remote patient monitoring, clinical decision support, and digital health data management in Japan.
The certification was issued by a Registered Certification Body (RCB) within the MHLW/PMDA framework (Certification No. 307AIBZX00024000; Issue date: August 6, 2025), validating the platform’s safety, efficacy, and compliance with Japan’s stringent medical device regulations under the Pharmaceuticals and Medical Devices Act.
Advanced SaMD Platform for Remote Healthcare Delivery
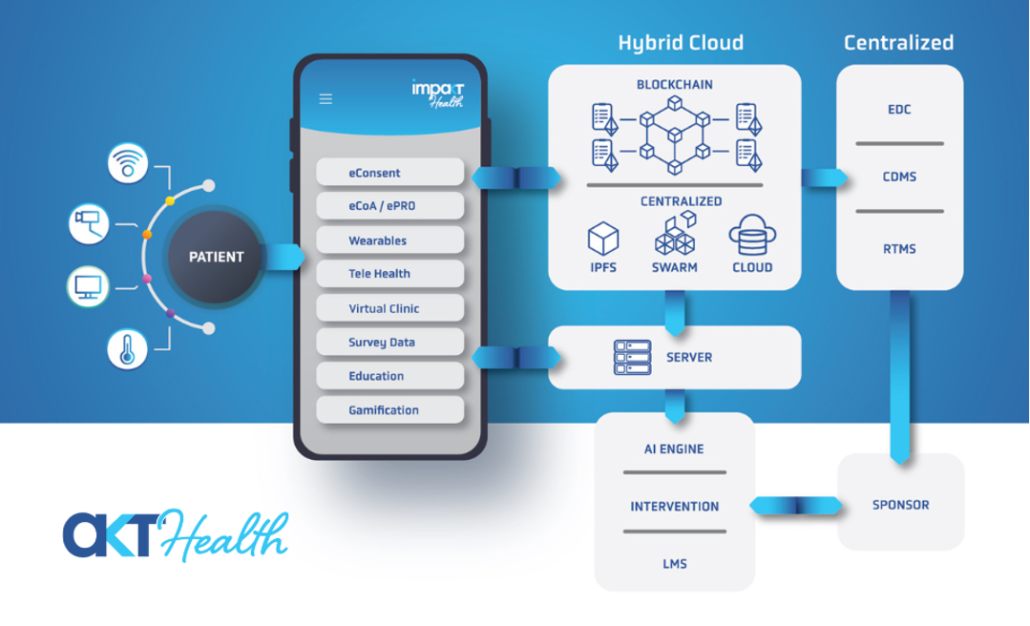
Impakt Health connects validated wearables, home sensors, and patient-reported inputs with clinician and research workflows. A hybrid architecture combines blockchain-anchored integrity with cloud performance to support secure, continuous data capture and near-real-time review across hospital-to-home care and decentralized/hybrid clinical trials (DCTs).
- Real-time remote monitoring: continuous trends for ECG, SpO2, body temperature, and blood pressure via validated devices
- Telehealth + patient app: guided onboarding, symptom check-ins, and virtual visits in one experience
- AI-assisted insights: individualized baselines highlight meaningful change for earlier outreach
- Blockchain-anchored data lineage: tamper-evident audit trails and provenance by design
- Interoperability: connectors to EHRs and study systems (EDC/CDMS/RTMS) for care and evidence generation
As healthcare systems adopt hospital-at-home models and outcome-based reimbursement, early insight into patient status becomes essential. Impakt Health continuously ingests physiologic signals, together with patient-reported inputs and helps teams act sooner, coordinate care across settings, and operate with audit-ready data, while minimizing patient burden through a single mobile experience simplifies device use, education, and remote check-ins, improving engagement without adding burden.
“This certification under Japan’s PMD Act confirms Impakt Health’s conformity to applicable safety and quality requirements for Class II devices,” said Aditya Kumar Tallapragada, President, AKT Health. “By unifying RPM, telehealth, and research-grade data capture, we give clinicians timely visibility and provide sponsors with high-fidelity evidence, without adding burden to patients.”
Designed for Real-World Operations
Role-based access, consent management, and data minimization protect privacy and governance. Each data event carries provenance; critical events are anchored to a blockchain ledger for a tamper-evident audit trail. Interoperability with EHRs and EDC/CDMS/RTMS enables a single data pipeline from care to research, with exports for statistical analysis. Built-in eCOA/ePRO and eConsent support DCT workflows.
Platform Overview
Impakt Health connects the patient app, clinician console, and research tools in one platform. Patients complete guided onboarding, pair approved sensors, and submit secure check-ins; clinicians view longitudinal dashboards, set thresholds and alerts, and document actions. Supported signals include ECG, SpO₂, body temperature, and blood pressure from validated devices. Under the hood, a hybrid architecture combines high-performance cloud services with a blockchain anchor for integrity and traceability, balancing scale with compliance in regulated environments.
Impakt Health is available to healthcare providers, research institutions, and life‑sciences sponsors in Japan. AKT Health is accepting partners for prioritized pathways in cardiometabolic, perioperative, and post-acute care. To request a demonstration or discuss deployment, contact info@akthealth.jp.
About Impakt Health
Impakt Health is AKT Health’s remote patient monitoring and digital clinical operations platform. It connects wearables, home devices, and patient reported outcomes with clinician dashboards and study systems, supporting continuous care, decentralized/hybrid clinical trials, and real‑world evidence generation.
About AKT Health Co., Ltd.
AKT Health is a data-driven life science consulting firm that empowers Medical and Commercial teams with AI-powered technology solutions, founded in 2019, operating across Japan, India, and the United States. We partner with pharmaceutical companies, biotechs, and healthcare organizations to transform complex healthcare data into actionable insights. Our integrated platform suite includes tools for KOL management, medical communications, commercial analytics, market access, and real-world evidence generation. By combining deep medical expertise with advanced analytics and digital innovation, we help clients optimize clinical development, enhance HCP engagement, accelerate market access, and demonstrate value to stakeholders. Our technology-enabled approach bridges the gap between scientific excellence and commercial success, enabling data-driven decisions across the entire product lifecycle.
Head Office: 1‑16‑8 Higashi, Shibuya‑ku, Tokyo, Japan
Website: https://akthealth.jp/ | LinkedIn
Media Contact: For inquiries regarding this announcement: info@akthealth.jp
Location: Japan,India,US,UAE,Nigeria
Hema Dubey | LinkedIn



